Over the weekend, I listened to an episode of The Ezra Klein Show that talked about how chaos is not just a byproduct of the Trump administration’s approach - it’s the strategy. Overwhelm people with a flood of executive orders, rule changes, and directives. Move fast. Create so many crises at once that it becomes impossible to track, let alone fight them all. The goal isn’t just to implement policy; it’s to exhaust opposition and break the systems designed to provide checks and balances. Steve Bannon, on a podcast explaining this exact strategy, called it “muzzle velocity.”
And when it comes to science and public health, the consequences of this approach are enormous. These executive actions threaten to dismantle critical public health programs, undermine scientific research, and erode trust in evidence-based policymaking. Rolling back environmental protections, restricting research funding, halting public health initiatives - each of these policies carries real risks for millions of Americans. Even if some of these efforts are ultimately blocked, the uncertainty alone creates instability in the institutions that safeguard public health, delaying progress and weakening response systems.
But here’s the thing: executive orders and agency directives still have to comply with the Constitution and the law. Presidents can’t unilaterally rewrite acts of Congress or override existing legal protections. That’s why we’ve already seen some of these efforts stall - like the sweeping order to halt all federal grant spending that came from the OMB’s two-page memo. A federal judge blocked it, and the administration was forced to withdraw the directive.
This is important to remember. The volume and speed of these actions are designed to make us feel powerless, but we aren’t. Many of these policies will face legal challenges. Some will be overturned. And understanding what’s happening, not just the headlines, but the mechanics of these orders, can help cut through the overwhelm.
So, let’s break it down. Here are the major actions Trump has taken in his first two weeks that directly impact science and public health: what they do, why they matter, and where legal challenges stand.
1. Withdrawal from the World Health Organization (WHO)
Type: Executive Order
What happened: The United States initiated the process to withdraw from the WHO, a move that takes one year to finalize. In the interim, federal health agencies, including the Centers for Disease Control and Prevention (CDC), have been directed to cease communication with the WHO.
Stated reasoning: The administration cited the WHO's handling of the COVID-19 pandemic and alleged undue political influence as primary reasons for withdrawal.
Why it matters: Withdrawing from the WHO weakens global and domestic health security by cutting off the U.S. from critical disease surveillance, pandemic preparedness, and public health research. The CDC relies on WHO data-sharing systems to track emerging threats like new COVID variants and avian flu, and losing access compromises our ability to respond effectively. This decision also disrupts international partnerships in vaccine distribution, disease prevention, and drug approvals, which could slow medical advancements and increase costs. Additionally, it undermines U.S. leadership in global health policy, leaving a power vacuum that other nations, like China, may fill. Beyond these immediate risks, withdrawing from the WHO reinforces distrust in public health institutions and weakens efforts to combat misinformation, ultimately harming long-term health outcomes. More information here.
Legal challenges: As of now, there are no reported lawsuits challenging this withdrawal. However, lawsuits are being considered, and Trump has already indicated that he may consider rejoining the WHO.
2. Communication Freeze at Federal Health Agencies
Type: Internal directive from HHS
What happened: Agencies such as the CDC, NIH, and the Food and Drug Administration (FDA) were instructed to pause all public communications, including reports like the Morbidity and Mortality Weekly Report (MMWR). This was a wide-sweeping order that included health advisories, scientific reports, website updates, and social media posts. Additionally, it required that any guidance, regulations, notices, or grant announcements receive approval from a presidential appointee before release.
Stated reasoning: The administration sought to control the narrative and ensure consistent messaging across agencies.
Why it matters: The communication freeze at federal health agencies has already had serious implications for public health. One of the most immediate and alarming effects is the disruption of the Morbidity and Mortality Weekly Report (MMWR), a critical publication from the CDC that provides real-time data on disease outbreaks, public health trends, and emergency health threats. The MMWR serves as a key resource for healthcare professionals, researchers, and policymakers, guiding decisions on disease prevention and response. Without it, critical information about flu activity, emerging infectious diseases, environmental health hazards, and vaccine effectiveness is not reaching the public or medical community in a timely manner. Additionally, the freeze has delayed public health guidance updates, scientific reports, and funding announcements, creating uncertainty and confusion in research and healthcare sectors.
Legal challenges: No current legal challenges. The freeze was set to last until Feb 1, but it does not appear to have been lifted as of now (Feb 3).
3. Halt on NIH Research Funding
Type: Internal directive from HHS
What happened: A temporary suspension was placed on the issuance of new research grants by the NIH, leading to delays in clinical trials and other research activities.
Stated reasoning: The administration aimed to review funding allocations to ensure alignment with its “priorities.”
Why it matters: I wrote a newsletter about the potential implications here. Briefly, the pause in funding disrupts scientific progress by stalling projects on diseases like cancer, Alzheimer’s, and rare genetic disorders that rely on continuous funding. Many research labs operate on tight grant cycles, so any disruption can lead to layoffs, loss of expertise, and even the termination of long-term studies. The delay also impacts drug development and public health innovations, as clinical trials for new treatments and vaccines may be postponed or canceled. Additionally, this uncertainty discourages young scientists from entering the field and may drive top researchers to seek funding in other countries, weakening the U.S.’s leadership in medical research. Cutting off NIH funding, even temporarily, threatens the infrastructure that underpins American scientific and medical advancements, with consequences that could last far beyond the duration of the freeze.
Legal challenges: No legal actions have been reported regarding this directive.
4. Pause all Federal Grant Funding
Type: OMB memo
What happened: A two-page memo from the Office of Management and Budget (OMB) instructed federal agencies to pause all new federal grant funding across multiple agencies, including research grants, public health initiatives, and social programs. The directive mandated that all pending and existing grants be reviewed to ensure alignment with the administration’s priorities and authorized political appointees to cancel already-approved grants if they were deemed inconsistent with those priorities.
Stated reasoning: The administration argued that the freeze was necessary to review federal spending and ensure taxpayer funds were allocated in ways that supported the administration’s policy goals.
Why it matters: The freeze immediately disrupted scientific research, public health programs, and education grants. Clinical trials faced delays, researchers experienced funding uncertainty, and essential public health initiatives, including nutrition assistance, disease prevention programs, and community health efforts, were put at risk. The directive introduced political influence over funding decisions, raising concerns about interference in evidence-based research and policy.
Legal Challenges: The memo was swiftly challenged in court by nonprofit organizations and public health advocates. A federal judge issued a temporary restraining order, blocking its implementation. Shortly thereafter, the Trump administration withdrew the directive.
5. Removal of Federal Webpages and datasets
Type: Administrative Action
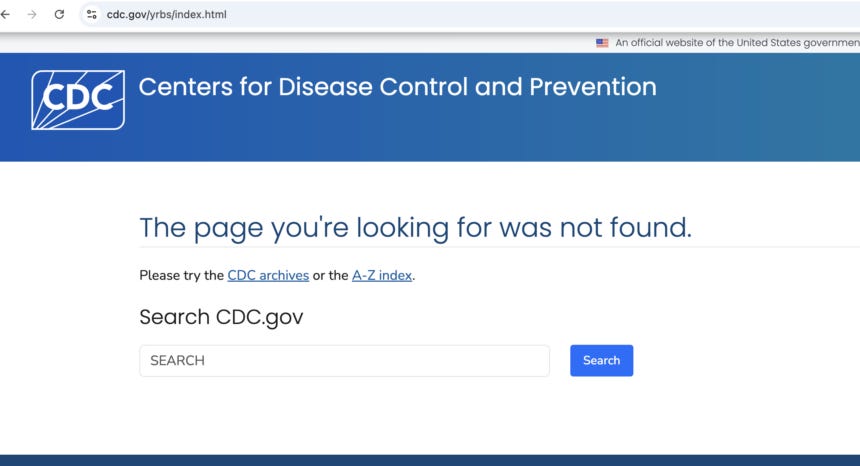
What happened: More than 8,000 federal webpages, including many public health and scientific resources, were taken offline. This included CDC datasets foundational to public health research and practice, such as the Youth Risk Behavior Surveillance System, as well as information about vaccines, veteran’s care, hate crimes, and scientific research. Additionally, ReproductiveRights.gov, a federal website providing information on reproductive healthcare access, was also removed.
Stated Reasoning: The administration did not provide an explicit justification, but similar actions in the past were framed as efforts to streamline government communications or remove materials that did not align with administration priorities.
Why it matters: The removal of these resources limits access to crucial public health data, which could hinder research, policy-making, and public awareness efforts. The disappearance of reproductive health resources may also make it harder for people to access accurate information about their rights and healthcare options.
Legal Challenges: Advocacy groups and researchers have raised concerns, but no lawsuits have been reported directly challenging these website removals.
6. Restrictions on Scientific Integrity in Federal Agencies
Type: Executive Order
What happened: This executive order imposes strict oversight on how federal scientists communicate their research. It requires that all public statements, publications, and presentations—including peer-reviewed studies, press releases, and congressional testimonies—undergo prior review and approval by political appointees within the respective agencies. The order also limits direct communication between federal scientists and the media, restricting unsanctioned interviews and public disclosures.
Stated reasoning: The administration claims the order is necessary to ensure that scientific messaging aligns with administration policies and priorities. The White House has framed it as a measure to prevent “misinformation” and maintain consistency in federal communication. Officials have also cited concerns over “gender ideology extremism,” suggesting that federal agencies have been used to promote ideological agendas rather than objective research.
Why it matters: This order threatens the integrity of federally funded science by centralizing control over research communication, making it easier for political appointees to suppress or alter findings that contradict the administration’s policy goals. By requiring approval for scientific publications, public statements, and media interactions, it creates a chilling effect that discourages researchers from pursuing politically sensitive topics such as climate change, vaccine safety, and environmental regulations. The restrictions also have far-reaching consequences for public health and safety. Agencies like the CDC and FDA rely on the ability to disseminate timely and transparent information to inform public health responses. Delays in research disclosures or public advisories could weaken responses to emerging health threats, increase public confusion, and erode trust in federal health agencies.
Legal challenges: Several scientific and legal advocacy groups have signaled plans to challenge the executive order in court.
7. Restrictions on Scientific Publications Using Banned Words
Type: Internal directive
What happened: Federal agencies, including the CDC and other public health and scientific institutions, were instructed to stop or delay the publication of research, reports, and other scientific communications that include certain words deemed politically sensitive by the administration. These words reportedly include terms such as gender, transgender, LGBT (lesbian, gay, bisexual and transgender) and nonbinary.
Stated reasoning: The administration argued that certain terminology reflects political bias and should be avoided in federally funded research and communications.
Why it matters: This directive poses a serious threat to scientific integrity, public health communication, and evidence-based policymaking. By restricting the use of certain terms, the administration effectively censors research on critical topics, including reproductive health, climate change, and marginalized communities. Researchers and public health officials may be forced to alter their language, potentially compromising the accuracy and clarity of their findings. This can have widespread consequences, from limiting the government’s ability to issue clear public health guidance to hindering research on pressing social and health issues. Additionally, the chilling effect of this directive could deter scientists from pursuing essential research topics altogether, further politicizing science and undermining public trust in federal research institutions.
Legal Challenges: None as of now, but they are expected.
8. Halt on U.S. HIV/AIDS Global Health Funding
Type: Executive Order
What happened: A temporary suspension was placed on foreign aid programs, including those under the President's Emergency Plan for AIDS Relief (PEPFAR), as part of a broader freeze on USAID funding.
Stated reasoning: The administration cited the need to review foreign aid spending to ensure it aligns with U.S. interests. Officials initially did not clarify whether PEPFAR and other life-saving health initiatives would be exempt, fueling widespread concern among global health organizations.
Why it matters: This jeopardizes decades of progress in HIV/AIDS treatment and prevention, particularly in low-income countries that rely on U.S. funding for antiretroviral therapy, prevention efforts, and maternal and child health programs. The sudden funding freeze has already led to service disruptions, increasing the risk of HIV treatment lapses that accelerate drug resistance and viral spread. Vaccine distribution and pandemic preparedness efforts are also affected, raising the likelihood of new disease outbreaks that could spread internationally.
Following backlash, the State Department later confirmed that PEPFAR is exempt under a waiver for life-saving humanitarian aid during the 90-day pause. However, confusion over implementation has left many clinics uncertain about future funding, forcing them to reduce services or turn away patients. If the freeze continues beyond the initial review period, it could trigger long-term setbacks in global HIV/AIDS efforts and weaken U.S. leadership in global health initiatives.
Legal Challenges: International organizations and advocacy groups are exploring legal avenues to challenge the suspension, arguing it violates U.S. foreign aid commitments and endangers public health.
9. Halting Limits on Per- and Polyfluoroalkyl Substances (PFAS) in Water
Type: Executive order
What happened: The administration halted plans to implement new federal limits on PFAS in drinking water. These chemicals, often referred to as "forever chemicals" due to their persistence in the environment and human body, are linked to a range of serious health risks. The halted policy would have set legally enforceable limits on PFAS levels in public water systems and required stronger monitoring and cleanup efforts in contaminated areas.
Stated reasoning: The administration justified the decision by stating that further study was needed to assess the economic impact of stricter PFAS regulations on industries that use these chemicals in manufacturing.
Why it matters: Halting the implementation of PFAS discharge limits may lead to continued environmental contamination, posing health risks to communities reliant on affected water sources. Without enforceable federal standards, the responsibility for regulating PFAS discharges may fall to individual states, potentially resulting in inconsistent protections nationwide.
Legal challenges: Environmental and public health advocacy groups have announced plans to challenge this order in court.
10. Reversal of Menthol Cigarette Ban
Type: Withdrawal of proposed Biden-era rule
What happened: The administration rescinded an FDA-planned ban on menthol cigarettes, which public health experts estimated could have saved over 650,000 lives.
Stated reasoning: The administration justified the reversal by citing concerns over personal freedom, government overreach, and potential economic impacts on small businesses and communities where menthol cigarette sales are concentrated.
Why it matters: Public health organizations have long advocated for banning menthol cigarettes, as studies show they are more addictive and disproportionately marketed toward Black Americans, exacerbating smoking-related health disparities. The decision to lift the ban allows tobacco companies to continue targeting vulnerable populations, potentially leading to increased rates of lung cancer, heart disease, and other smoking-related illnesses. Research has shown that menthol cigarettes make it easier to start smoking and harder to quit, which is why they have been particularly harmful to marginalized communities. Without restrictions, smoking-related deaths and healthcare costs are likely to remain high, further straining public health systems.
Legal Challenges: While advocacy groups and public health organizations have strongly condemned the decision, no legal actions have been reported against this reversal.
11. Rescission of Prescription Drug Pricing Policy
Type: Executive Order
What happened: The order reversed a Biden-era policy aimed at lowering prescription drug costs by allowing Medicare to negotiate drug prices and limiting price hikes on essential medications. The rescinded policy was designed to curb excessive pharmaceutical costs, particularly for seniors and low-income individuals who rely on Medicare and Medicaid.
Stated reasoning: The Trump administration justified the reversal by claiming that price controls stifled pharmaceutical innovation, discouraging companies from investing in new drug development, and that government-imposed pricing measures disrupted the free market and led to potential drug shortages.
Why it matters: This reversal could result in higher prescription drug costs for millions of Americans, particularly those on Medicare and Medicaid. Patients who rely on life-saving medications for chronic conditions such as diabetes, heart disease, and cancer may face increased financial burdens. Additionally, without cost controls, pharmaceutical companies have more freedom to raise prices, potentially exacerbating healthcare affordability issues and reducing medication adherence, which could worsen health outcomes over time.
Legal challenges: There are no known legal challenges to this executive order at this time, but public health advocates and patient advocacy groups have voiced strong opposition.
12. Affordable Care Act (ACA) Rollbacks
Type: Executive Order
What happened: The order implemented measures aimed at reducing access to insurance through the ACA. These measures included shortening the enrollment periods for ACA marketplaces and reducing funding for outreach programs designed to educate and assist individuals in obtaining health insurance coverage.
Stated reasoning: The administration emphasizing a desire to decrease government intervention in the healthcare sector. By promoting market-based solutions, the administration aimed to foster competition among insurance providers, which they argued would lead to more personalized and cost-effective healthcare options for consumers.
Why it matters: Critics argue that these rollbacks could lead to an increase in the number of uninsured individuals. Shortened enrollment periods and reduced outreach funding may result in lower awareness and understanding of available insurance options, particularly among vulnerable populations. This decrease in coverage could lead to poorer health outcomes, as individuals without insurance may forgo preventive care or delay seeking medical attention.
Legal challenges: Several states and advocacy groups have filed lawsuits challenging ACA rollbacks. However, lawsuits for this specific action are currently unknown.
12. Overhaul and Potential Shutdown of USAID
While writing this newsletter, a lot is happening regarding the overhaul and potential shutdown of USAID. I am including it here to give context about what we know so far.
Type: Administrative Actions and Proposed Structural Changes
What is happening: In late January 2025, the Trump administration implemented a near-total freeze on USAID foreign aid programs, halting approximately $43 billion in assistance to over 130 countries. Secretary of State Marco Rubio announced plans to merge USAID into the State Department, arguing that the agency had become inefficient and misaligned with U.S. priorities. Elon Musk, appointed to lead the Department of Government Efficiency (DOGE), has publicly criticized USAID as a “black hole of corruption” and signaled that a full shutdown is under serious consideration. Without prior notice, USAID employees were locked out of their headquarters, senior officials were placed on administrative leave, and critical funding for disaster relief, global health, food security, and humanitarian programs was abruptly cut off.
Stated reasoning: The administration claims that integrating USAID into the State Department will eliminate waste, reduce the federal workforce, and ensure that foreign aid directly benefits U.S. interests. Musk has argued that USAID is ineffective and plagued by corruption, with too much aid being misused or failing to reach its intended recipients.
Why it matters: The USAID funding freeze is already disrupting life-saving aid worldwide. Food assistance programs have been suspended, leaving millions at risk of malnutrition. Vaccine distribution for measles, malaria, and cholera has stalled, increasing the risk of deadly outbreaks. Disaster relief efforts have ground to a halt, leaving survivors of hurricanes, earthquakes, and refugee crises without U.S. assistance.
Beyond the humanitarian crisis, dismantling USAID would fundamentally reshape U.S. foreign policy. The agency has long been key to stabilizing fragile regions and countering China and Russia’s influence. Cutting off aid risks creating a power vacuum that adversaries will exploit, while also damaging U.S. credibility, weakening global health security, and fueling migration and instability.
The move has drawn a lot of backlash. Lawmakers, aid groups, and international partners have condemned it as reckless. Democrats are exploring legal challenges, arguing the president lacks authority to freeze congressionally approved aid. If USAID is dismantled, it would mark the largest rollback of U.S. humanitarian aid in modern history, undermining America’s role in global health and crisis response.
Final Thoughts
It took me twice as long as I expected to sort through the executive orders and actions from the first two weeks of the Trump administration and write this up - and that’s just the ones directly impacting public health. The sheer volume, the overlapping policies, and the lack of clear information on exactly what is impacted and how make it almost impossible to track. But that’s the point. The confusion isn’t a side effect - it’s a strategy. When people feel overwhelmed, they disengage. When the news cycle moves too fast to keep up, opposition gets spread thin. But as disorienting as this all is, the antidote to confusion is clarity, and the antidote to overwhelm is action. The more we understand what’s happening, the better we can push back effectively. That means staying informed, supporting organizations fighting these rollbacks, and keeping the pressure on lawmakers and courts where it matters. Chaos is the strategy. Refusing to look away is ours.
If you're wondering what to do next, download the 5 Calls app - it makes it easy to contact your senators and representatives with pre-written scripts and phone numbers, so you can take action in just a few minutes.